Mineral fertilizers are preparations for application to the soil that contain micro- and macroelements. The fertilizer mass calculator helps determine their quantity. Annual application of mineral fertilizers maintains soil fertility and stable yields. With one caveat - if you add minerals correctly. To quickly calculate how much substance you need to measure, use the Fertilizer Mass Calculator.
What are the rules for applying autumn and spring fertilizers? What are the differences, is there compatibility and how to determine the deficiency of one or another substance? This is discussed in our article.
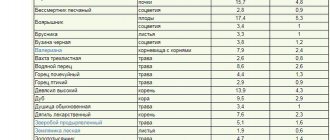
Mineral fertilizers
Minerals restore the balance of soil elements. They are called chemical, although the sources for their production can be natural components. For example, saltpeter, natural apatites and phosphites. Chemical production plants, for example, coke plants, are also a source of complex fertilizers.
Chemical mineral fertilizers are divided into nitrogen, phosphorus, and potassium. There are also microfertilizers - compositions with small doses of microelements (for example, boron, manganese, zinc).
Note: phosphorus, nitrogen, potassium are the main elements of plant nutrition.
The compositions are divided into single-component and complex fertilizers - containing several substances. Available in the form of powders, granules, and liquids.
Compatibility table for various chemical elements that make up fertilizers; to determine their quantity, use the Mineral Fertilizer Mass Calculator.
How to measure organics
Organic fertilizers are applied during soil preparation for planting and in the preparation of soil mixtures for seedlings of vegetables, flowers, and ornamental crops. The volumes of humus, peat, compost, soil, sawdust, and ash are not small.
Top 10 dangerous fertilizers allowed in Russia Fertilizers allowed for use in Russia are divided into 2 large classes: organic and mineral. The first...
Their norm is measured with a 10 liter bucket, which includes:
- 13 kg of dry river sand;
- 12 kg of slurry;
- 12 kg of turf soil;
- 10 kg of compost;
- 9 kg sawdust (dry);
- 8 kg of manure, humus, leaf soil;
- 6 kg of dry chicken manure;
- 6.5 kg fluff;
- 5 kg of ash, dry peat.
Nitrogen fertilizers
The source of mineral fertilizers with nitrogen is synthetic ammonia. Natural sources of nitrogen are organic matter and manure, also with large amounts of ammonia. When decomposed, ammonia is converted to release nitrogen, which is absorbed by plants.
Chemical nitrogen is a substance that is easily absorbed by the root system. Nitrogen fertilizers are one of the most popular fertilizers for the garden. In stores you can find the following list of nitrogen complexes:
- Ammonia mineral fertilizers contain nitrogen in the form of ammonia. They dissolve well in water and quickly reach the plants. Note: nitrogen is better absorbed from ammonium compounds than from nitrate compounds.
- Nitrate mineral fertilizers are various salts of nitric acid, sodium, calcium, potassium nitrate. They dissolve less easily in water and require heating.
- Ammonium nitrate - ammonium nitrate. Contains two forms of nitrogen (ammonia and salt). You also need hot water to dissolve them.
- Amide - urea (another name is urea) - a product of protein utilization, decomposes with the release of nitrogen.
Important: When nitrogen is applied in excess, plants accumulate dangerous toxins - nitrates. In addition, only 50% of nitrogen from fertilizing is absorbed by plants, the other half is “lost.” Partially washed out by soil waters and partially evaporated into the atmosphere. When evaporated, a greenhouse gas is formed - nitrogen oxide N2O.
What else to consider?
- Due to the high rate of evaporation, nitrogen mineral fertilizers must be sealed and buried. And do this immediately after making it.
- For the same reason, nitrogen fertilizers for the garden and vegetable garden are stored in tight plastic sealed packaging without access to air.
The rate of nitrogen application depends on the type of plant and time of year.
Ammonium sulfate
(NH4)2SO4 is a powder obtained from ammonia and sulfuric acid. It is a by-product of the coke and metallurgical industries. It is characterized by low toxicity and is used by the food industry as an additive E517 (salt substitute, increases the volume of flour and bakeability of bread) and for water chlorination.
It is slightly hygroscopic and odorless. Approved for use at any time of the year.
In agriculture it is most effective on uncultivated soils. On dug up soils, ammonium quickly turns into nitrate form and loses its effectiveness.
It increases the acidity of soil compounds, so it should not be used frequently to avoid severe acidification of the soil.
Ammonium sulfate application rates, for ease of calculation, use the Mineral Fertilizer Mass Calculator.
| Application rate, g/m2 | |
| Vegetables | |
| potato | 30-50 |
| cucumbers | 20-40 |
| Beets, carrots | 25-30 |
For foliar feeding or watering, for seedlings, ammonium sulfate is dissolved, maintaining a ratio of 10 g. (1 tbsp) per bucket of water (10 l).
Ammonium nitrate
NH4NO3 is obtained from ammonia and nitric acid. The second name for ammonium nitrate is ammonium nitrate. The substance is used to produce explosives, so in a number of countries it is restricted for free sale.
Important: ammonium nitrate should not be stored in the sun. Heating above 32°C may cause an explosion.
Dissolves with heat absorption. Therefore, to dissolve the saltpeter powder, it is necessary to heat the water and add the powder in small portions.
Ammonium nitrate application rates
| Application rate, g/m2 | |
| Undepleted soils | 20–30 |
| Depleted soils | 35–50 |
| Autumn digging | from 5 to 20, namely: |
| Fruit trees | 15–20 |
| Shrubs | 10-15 |
| Vegetables | 5-10 |
| Roots | 5–7 |
| Planting seedlings in spring | 4-7 |
For foliar feeding or spraying, dissolve 50 g of saltpeter per 100 liters of water and use it per 100 sq.m. Plants and trees are fed with a solution of nitrate 5-6 days after flowering.
Urea (urea)
Urea or CH₄N₂O is produced from ammonia and carbon dioxide. For agriculture, grade B urea powder is used.
As a top dressing, urea is applied in the fall (embedded into the soil), in the spring (for winter crops), and throughout the season (liquid fertilizer is used for watering and spraying).
Note: when sprayed, urea does not cause leaf burn.
When applied to the soil, urea is embedded to a depth of 3-4 cm. The timing of spring incorporation is 1-2 weeks before planting. After incorporating the urea powder, water the soil.
Urea application rates
| Application rate, g/m2 | |
| Vegetables | |
|
|
|
|
|
|
| Fruit trees, berry bushes | 20-30 |
For foliar feeding, dissolve 200 g of urea powder per 10 liters of water.
You will learn how to properly fertilize with urea from the video.
Signs of nitrogen deficiency
The lack of nitrogen is clearly visible on tomatoes, potatoes, and cucumbers. At an early stage of development, stunted growth, thin stems, and a small number of inflorescences are observed. When leaves and stems appear, foliage that is too light becomes noticeable, and the lightening begins with the veins. If the necessary measures are not taken in time, the leaves may fall off.
Our experiment
We decided to conduct our own experiment and weigh how much urea fits in a tablespoon, teaspoon, or glass.
It turned out that 1 tablespoon contains 15 g of urea :
1 teaspoon contains 5 grams:
A matchbox contains 14 grams of urea.
It turned out that our indicators completely coincided with the approximate measurements only in a matchbox. Compare:
In a tablespoon: 10-12 g (we have 15 g), In a teaspoon: 3-4 g (we have 5 g), In a matchbox: 13-15 g (we have 14 g)
The fact that the obtained indicators partially coincided with the approximate standards once again proves that spoons differ in volume, everyone scoops in their own way (some with a heap, some without). Therefore, it is better to take your own measurements and use them. But if this is not possible, we hope our tips will be useful to you.